Frankliniella williamsi Hood, 1915
Thripinae, Thripidae, Terebrantia, Thysanoptera
Figures
Fig. 1: 8-segmented antenna, segments III and IV with forked sense cone, terminal segments VI-VIII
Fig. 2: Head dorsal with ocellar triangle
Fig. 3: Pronotum
Fig. 4: Meso- and metanotum
Fig. 5: Fore- and hind wing, fore wing distal region
Fig. 6: Meso- and metasternum
Fig. 7: Sternites II and III
Fig. 8: Sternite VII
Fig. 9: Tergite VIII with posteromarginal comb
Introduction and recognition
Frankliniella williamsi breeds in the leaf axils of young plants of maize and other grasses. Both sexes fully winged; body color yellow, antennal segments VII & VIII and distal half of VI brown; fore wings pale. Antennae 8-segmented; segments III & IV with forked sense cone, segment VIII about twice the length of antennal segment VII (Fig. 1). Head wider than long; 3 pairs of ocellar setae present, pair III about as long as distance between hind ocelli and arising just within anterior margins of ocellar triangle; postocular setae pair I present, pair IV distinctly shorter than distance between hind ocelli (Fig. 2). Pronotum with 4-5 pairs of elongate setae (1 pair anteromarginally, 1 pair anteroangularly, 2 pairs posteroangularly and 1 pair of moderately elongate posteromarginal submedian setae) (Fig. 3). Metanotal median area transverse at anterior and with irregular equiangular or longitudinal reticulations on posterior half; median setae longer than lateral setae and arising at anterior margin; campaniform sensilla present (Fig. 4). Mesofurca with spinula (Fig. 6). Mid and hind tarsi 2-segmented. Fore wing with 2 complete rows of veinal setae (Fig. 5). Tergites V-VIII with paired ctenidia laterally, on VIII anterolateral to spiracle; posteromarginal comb on VIII with long and fine, closely spaced microtrichia on broadly triangular bases (Fig. 9). Sternites III-VII without discal setae, except sternite II with 1 or 2 long discal setae medially (Fig. 7); median setae of sternite VII arising at or close to posterior margin (Fig. 8).
Male similar to female but smaller; tergite VIII with complete comb of long slender microtrichia, IX with median pair of setae shorter than lateral pair; sternites III-VII with small oval glandular area, sternite II with 1 or 2 discal setae medially, sternite VII with toothed craspedum on posterior margin.
Taxonomic identity
Species
Frankliniella williamsi Hood, 1915
Taxonomic history
Frankliniella spinosa Moulton, 1936
Frankliniella flavens Moulton, 1928
Common name
Corn thrips
Maize thrips
Present taxonomic position
Family: Thripidae Stephens, 1829
Subfamily: Thripinae (Stephens) Karny, 1921
Genus: Frankliniella Karny, 1910
Genus description
The genus Frankliniella Karny, 1910
This genus is mainly known from the New World and contains about 230 species, many of them from the Neotropics (Mound & Marullo 1996, Cavalleri & Mound 2012). Some species are widely known as crop pests - Frankliniella occidentalis, Frankliniella intonsa, Frankliniella schultzei (all of them vectors of tospoviruses) and Frankliniella williamsi. The members in this genus are sometimes quite difficult to separate from one another and the classification has been in flux with many species later synonymized in association with color variations. They mostly have 3 pairs of well developed ocellar setae, 8-segmented antennae with segments III and IV having forked sense cones, usually 4-5 pairs of elongate pronotal setae (1 pair anteromarginally, 1 pair anteroangularly, 2 pairs posteroangularly, and 1 pair of moderately elongate posteromarginal submedian setae S2 which are longer than median seta S1), metanotal median setae arising at anterior margin, when present wings with complete rows of setae on the wing veins, paired ctenidia laterally on tergites V-VIII with those on VIII anterolateral to the spiracles, no discal setae on sternites, and the males are generally smaller and paler than the females (Mound & Marullo 1996; Stannard 1968).
Species description
Typical key character states of Frankliniella williamsi
Coloration and body sculpture
Body color: mainly pale to yellow, or with some darker markings
Surface of head, pronotum and fore legs: without obvious or with weakly reticulate sculpture
Antennae
Form of sense cones on antennal segments III and IV: emergent and forked on segments III and IV
Number of antennal segments: 8
Antennal segment I: without any setae on dorsal apical margin
Antennal segment II: without an exceptionally long seta at the inner apex
Antennal segment II shape: symmetric
Antennal segment III shape: symmetric
Length of antennal segment III and IV: antennal segment III similar in length to segment IV
Forked sense cone on antennal segment IV: scarcely extending beyond base of segment V
Antennal segment IV and V: without a hyaline ring near the base
Antennal segment VI bears: not a remarkably dagger-shaped sensorium
Antennal segment VIII length: about twice the length of antennal segment VII
Shape of pedicel on antennal segment III: simple
Head
Distance between bases of ocellar setae III: greater than width of first ocellus
Head: not prolonged in front of compound eyes
Pair of major postocular seta: subequal to other postoculars and distinctly shorter than distance between hind ocelli
Ocellar setae I: present
Length of ocellar setae II: shorter than setae III
Ocellar setae III: arising within ocellar triangle anterior to tangent of anterior margin of hind ocelli
Ocelli: present
Ocellar setae III length: about as long as distance between hind ocelli
Length of postocular setae: not alternating short and long setae
Number of ocellar setae: 3
Prothorax
Number of pairs of anteromarginal minor setae: 2-3
Number of pairs of long anteroangular setae: 1-2
Number of pairs of long posteroangular setae: 2
Number of pairs of elongate pronotal setae: 4-5
Number of pairs of posteromarginal minor setae: 4-5
Pronotal blotch or internal apodeme: absent
Pronotum shape: broadly rectangular
Pronotum posteromarginal/posteroangular setae: S2 longer than S3, not equal in length
Mesothorax
Mesosternal furca: with median spinula
Metathorax
Metanotal campaniform sensilla: present
Metanotal median setae: S1 at anterior margin
Metanotum with dominant sculptured triangle medially: absent
Metasternal furca: without spinula
Sculpture of metanotum median area: transverse at anterior, but irregular longitudinal or equiangular reticulations on posterior half
Shape of metathoracic furca: transverse, V-shaped
Metanotal median setae length: longer than lateral metanotal setae
Wings
Fore and hind wings: present, more than half as long as abdomen (macropterous)
Fringe cilia arising: from sockets
Fore wing veins: present
Fore- and hind wing surface: covered with microtrichia
Apex of fore wing: with prominent terminal setae
Fore wing anterior margin (costal vein): with setae and cilia but cilia longer than setae
Fore wing costal fringe cilia: arising at anterior margin of wing
Fore wing first vein: distinct from costal vein
Fore wing first vein setal row: complete, with setae closely and uniformly spaced
Fore wing second vein setal row: complete, setae uniformly spaced
Fore wing shape: mainly parallel sided or margins run continuously towards each other
Fore wing surface: not reticulate
Fringe cilia on posterior margin near apex: distinctly wavy (undulated)
Length of fore wing costal setae at middle of wing: longer than half of median wing width
Shape of fore wing apex: with mainly posterior margin curved to join anterior margin
Fore wing extreme apex color: pale
Fore wings: uniformly pale or weakly shaded
Legs
Fore tibia: not prolonged around fore tarsus
Mid and hind tarsi: with two segments
Color of fore tarsi: pale or yellow, sometimes apical shaded or brown
Abdomen
Ovipositor curved: downwards
Pleurotergites: not covered in microtrichia
Sternite II: with marginal setae and few discal setae
Sternites IV, V and VI: with marginal setae but no discal setae
Sternite VII: with marginal setae but no discal setae
Surface of lateral thirds of abdominal tergites: without regular rows of fine microtrichia
Tergites II to VII median setal pair: no more than 0.3 as long as median length of tergite
Craspedum on tergites IV to VI: absent
Tergites IV and V median setal pair: shorter than distance between their bases
Tergites V to VII: with ctenidia laterally
Craspedum on tergite VIII: without craspedum medially and toothlike microtrichia laterally
Tergite VIII ctenidia: anterolateral to spiracle
Tergite VIII posteromarginal comb of microtrichia: present and complete medially
Tergite VIII shape of posteromarginal microtrichia: long on broadly triangular bases or long, slender and regular on broadly triangular bases
Tergite X: not tubular, longitudinally incomplete
Setae on abdominal tergite X: all setae slender

Similar or related species
Compared to other members of the genus, Frankliniella williamsi has 1 or 2 median discal setae in addition to marginal setae on the sternite II (Frankliniella borinquen, Frankliniella occidentalis and Frankliniella schultzei without discal setae on sternite II), postocular setae IV distinctly shorter than distance between hind ocelli (in Frankliniella borinquen as well as Frankliniella schultzei the length of postocular setae IV are about as long as distance between hind ocelli; in Frankliniella occidentalis postocular setae IV longer), tergite VIII always with a complete comb of long and fine, uniform spaced microtrichia on broadly triangular bases (in Frankliniella borinquen and Frankliniella occidentalis the comb on tergite VIII is complete, but in Frankliniella borinquen with short microtrichia arising from triangular bases and in Frankliniella occidentalis with short or long microtrichia arising from triangular bases; in Frankliniella schultzei posteromarginal comb of microtrichia weakly developed or complete absent). Frankliniella williamsi as well as Frankliniella occidentalis and Frankliniella borinquen with ocellar setae III arising on anterior margin of or just within anterior margin of ocellar triangle, and campaniform sensilla on metanotum normally present (only Frankliniella schultzei with ocellar setae III arising very close together between anterior margins of hind ocelli, and without metanotal campaniform sensilla). Frankliniella williamsi as well as Frankliniella occidentalis and Frankliniella schultzei have a simple pedicel of antennal segment III, and an antennal segment VIII that is longer than segment VII (only Frankliniella borinquen has the pedicel of antennal segment III swollen, with edged ring surmounted by a distinctive swelling and a slightly flared collar, and antennal segment VIII equal in length to or shorter than segment VII). In Frankliniella williamsi and Frankliniella schultzei ocellar setae III on head are about as long as distance between hind ocelli (in Frankliniella occidentalis ocellar setae III are about as long as distance between external margins of hind ocelli; in Frankliniella borinquen as long as distance between midpoint of hind ocelli).
Species of the genus Frankliniella are similar to species of Thrips, Stenchaetothrips, Microcephalothrips abdominalis, Larothrips dentipes and Fulmekiola serrata, in having tergites V-VIII which bear a pair of ctenidia laterally, but in these species ctenidia of the tergite VIII arranged posteromedial to the spiracle, whereas species of Frankliniella have ctenidia on tergite VIII placed anterolateral to the spiracle.
Biology
Life history
As with other thrips species the life cycle from egg to adult is dependent on temperature. The full cycle can take less than one week to over a month and adults may live for more than one month producing several generations in one year depending on seasonal weather (Lewis 1973).The life-cycle from egg - adult at 24°C and 86% RH ranged from 23-28 days. Fecundity ranged from 58.3 - 64.9 eggs/female. Unmated females produced fewer eggs and offsprings were all male (Wechakit and Ketavan, 2010).
Host plants
Mainly Poaceae, also other crops and weeds.
Crops: babycorn, beans (French beans, peas), capsicum, cassava, chillies, coriander, maize, onion, red gam, rice, sorghum, wheat. soy bean, peanut and cotton.
Weeds: Bidens pilosa, Tithonia diversifolia.
This species was found to infest babycorn intercropped with French beans in Kenya (Nyasani et al. 2012).
Vector capacity
Maize chlorotic mottle virus. MCMV is the only species in the genus Machlomovirus (family Tombusviridae) and Frankliniella williamsi is one among the vectors of this disease. It transmits the disease in a non-persistent manner (Nelson et al. 2011).
Damage and symptoms
Feeding on young leaves results in streaks on the leaves of maize.
Detection and control strategies
-
Additional notes
-
Biogeography
Widespread in tropical and subtropical countries around the world: Asia, Australia, New Zealand, Central and South America, North America. Kenya and Uganda.
African countries where Frankliniella williamsi has been reported
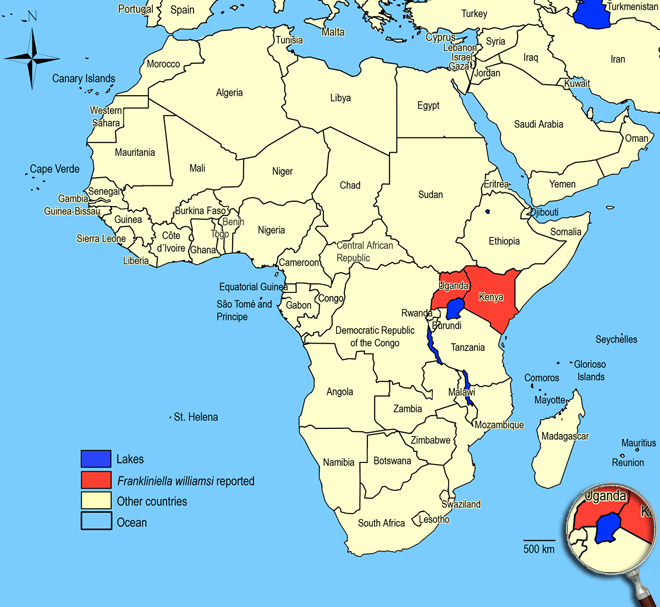
Occurence of Frankliniella williamsi in East Africa
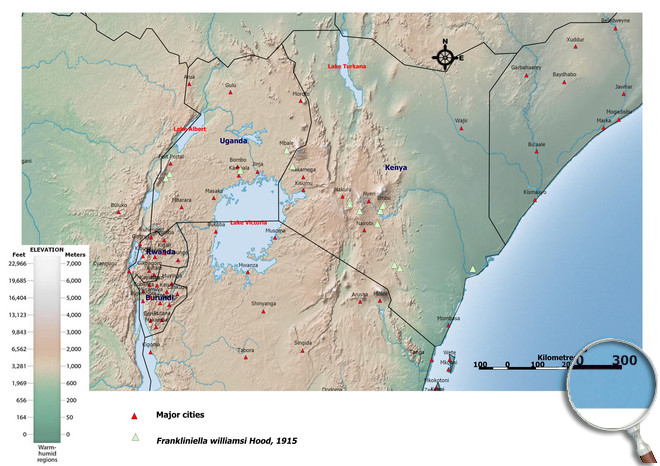
Please click here for survey sites of all observed thrips species of Kenya, Tanzania and Uganda.
Click here for locations of Frankliniella williamsi in parts of East Africa.

Bibliography
Bhatti JS (2006). The classification of Terebrantia (Insecta) into families. Oriental Insects. 40 (1): 339-375
Cach NT, Lenis JI, Perez JC, Morante N, Calle F & Ceballos H (2006). Inheritance of useful traits in cassava grown in subhumid conditions. Plant Breeding. 125 (2): 177-182
Cavalleri A & Mound LA (2012). Toward the identification of Frankliniella species in Brazil (Thysanoptera, Thripidae). Zootaxa 3270: 1-30
Hood JD (1915). Descriptions of new American Thysanoptera. Insecutor Inscitiae Menstruus. 3 (1-4): 1-40
Jiang XQ, Meinke LJ, Wright RJ, Wilkinson DR & Campbell JE (1992). Maize chlorotic mottle virus in Hawaiian-grown maize: vector relations, host range and associated viruses. Crop Protection. 11 (3): 248-254
Lewis T (1973). Thrips: their biology, ecology and economic importance. Academic Press Inc., London Ltd., 349 pp
Lewis T (1997). Thrips as crop pests. CAB International, Wallingford, 740 pp
Moritz G (2006). Thripse. Pflanzensaftsaugende Insekten, Bd. 1, (1. Auflage). Westarp, Hohenwarsleben, 384 pp. ISBN-13: 978 3 89432 891 7
Moritz G, Morris DC & Mound LA (2001). ThripsID - Pest thrips of the world. ACIAR and CSIRO Publishing Collingwood, Victoria, Australia, CDROM ISBN 1 86320 296 X
Moritz G, Mound LA, Morris DC & Goldarazena A (2004). Pest thrips of the world - an identification and information system using molecular and microscopical methods. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN 1 86499 781 8
Moritz G, O'Donnell C & Parrella M (2009). Pest thrips of North America. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN-13: 978 1 86499 940 2
Moulton D (1928). Thysanoptera of the Hawaiian Islands. Proceedings of the Hawaiian Entomological Society. 7 (1): 105-134
Moulton D (1936). New American Thysanoptera. Bulletin of the Brooklyn Entomological Society. 31 (2): 61-65
Mound LA (2002). The Thrips and Frankliniella genus groups: the phylogenetic significance of ctenidia, pp. 379-386. In Marullo R & Mound LA [eds.], Thrips and tospoviruses: Proceedings of the 7th International Symposium on Thysanoptera. Australian National Insect Collection, Canberra
Mound LA & Kibby G (1998). Thysanoptera: An identification guide, (2nd edition). CAB International, Wallingford and New York, 70 pp
Mound LA & Marullo R (1996). The thrips of Central and South America: An introduction (Insecta: Thysanoptera). Memoirs on Entomology, International, Vol. 6. Associated Publishers, Gainesville, 487 pp
Nelson S, Brewbaker J & Hu J (2011). Maize Chlorotic Mottle. Plant Disease 79: 1-6
Nickle DA (2003). A checklist of commonly intercepted thrips (Thysanoptera) from Europe, the Mediterranean, and Africa at U.S. ports-of-entry (1983-1999). Part I. Key to genera. Proceedings of the Entomological Society of Washington. 105 (1): 80-99
Nickle DA (2004). Commonly intercepted thrips (Thysanoptera) from Europe, the Mediterranean, and Africa at U.S. ports-of-entry. Part II. Frankliniella Karny and Iridothrips Priesner (Thripidae). Proceedings of the Entomological Society of Washington. 106 (2): 438-452
Nyasani JO, Meyhöfer R, Subramanian S & Poehling H.-M (2012). Effect of intercrops on thrips species composition and population abundance on French beans in Kenya. Entomologia Experimentalis et Applicata 142: 236-246
Palmer JM, Mound LA & du Heaume GJ (1989). 2. Thysanoptera, 73 pp. In Betts CR [ed.], CIE Guides to insects of importance to man. CAB International, Wallingford, Oxon, UK
Sites RW, Chambers WS & Nichols BJ (1992). Diel periodicity of thrips (Thysanoptera, Thripidae) dispersion and the occurrence of Frankliniella williamsi on onions. Journal of Economic Entomology. 85 (1): 100-105
Stannard LJ (1968). The thrips, or Thysanoptera, of Illinois. Illinois Natural History Survey Bulletin. 29 (4): 214-552
Wechakit D & Ketavan C (1975). Biological and morphological studies on the corn thrips, Frankliniella williamsi Hood (Thysanoptera: Thripidae). Kasetsart Journal 9(2): 133 - 141
----
Web links
Mound´s Thysanoptera pages
Thysanoptera Checklist
ICIPE Thrips survey sites
UNI Halle & Thrips sites
Thrips of California